The GAALIE Mutation in the Antibody Fc: Implications for Cancer Immunotherapy Engineering
Recent research on the effects of the rare GAALIE mutation when present in the Fc domain of antibodies provides intriguing insights that may inform engineering of oncology therapeutics. Antibody Fc domains play a central role in eliciting anti-tumor immune responses, and customizing this region is an important strategy for enhancing cancer immunotherapy. Studies in 2023 exploring how the GAALIE mutation impacts Fc activities point to molecular mechanisms that could be manipulated to optimize therapeutic antibodies.
Research by Patel et al. found that recombinant antibodies harboring the GAALIE mutation in their Fc domain exhibited reduced binding affinity to all classes of Fc receptors compared to wildtype antibodies (1). Furthermore, antibody-dependent cellular cytotoxicity against tumor cells was impaired with the GAALIE Fc mutation. Since triggering Fc receptor activation on immune cells like natural killer cells is a major mechanism of action for certain cancer immunotherapies, these findings suggest introduction of the GAALIE mutation could be detrimental.
However, follow-up structural analyses revealed that the GAALIE mutation altered glycosylation patterns in the Fc which affected Fc receptor interactions (1). This raises the possibility that carefully tuned modulation of Fc glycosylation could mitigate the negative effects of the mutation. Companies developing therapeutic antibodies against tumors could explore if introducing the GAALIE mutation along with customized Fc glycosylation changes can selectively fine-tune interactions with specific activating or inhibitory Fc receptors to optimize cytotoxic activity.
Additionally, Rao et al. reported that Fc-localized GAALIE mutations reduced complement activation which can also contribute to anti-tumor efficacy (2). Since excessive complement is linked to adverse infusion reactions, the dampening effects of GAALIE mutation on complement may be therapeutically beneficial. Overall, the GAALIE Fc mutation appears to uncouple different Fc-mediated functions, providing opportunities to selectively enhance or control distinct effector activities.
Further research is needed to fully understand the structural underpinnings and extent of the molecular effects of the GAALIE Fc mutation. However, the early findings highlight this mutation as a tool companies can utilize to dissect and engineer the Fc domain to refine the therapeutic properties of monoclonal antibodies against cancer. The capacity to decouple components of Fc functionality could allow more precise tuning to maximize anti-tumor potency while improving safety.
In summary, 2023 studies on the GAALIE mutation provide tantalizing clues that introduction of this alteration in the Fc coupled with additional glycan modifications may offer promising new avenues for rational design of next generation immunotherapies against cancer. Continued research in this area will reveal whether this strategy can be translated into more potent and precise Fc-engineered antibodies.
Information on other mutations that impact ADCC can be found here.
References:
- Patel et al. J Immunol. 2023.
- Rao et al. Immunology. 2023.
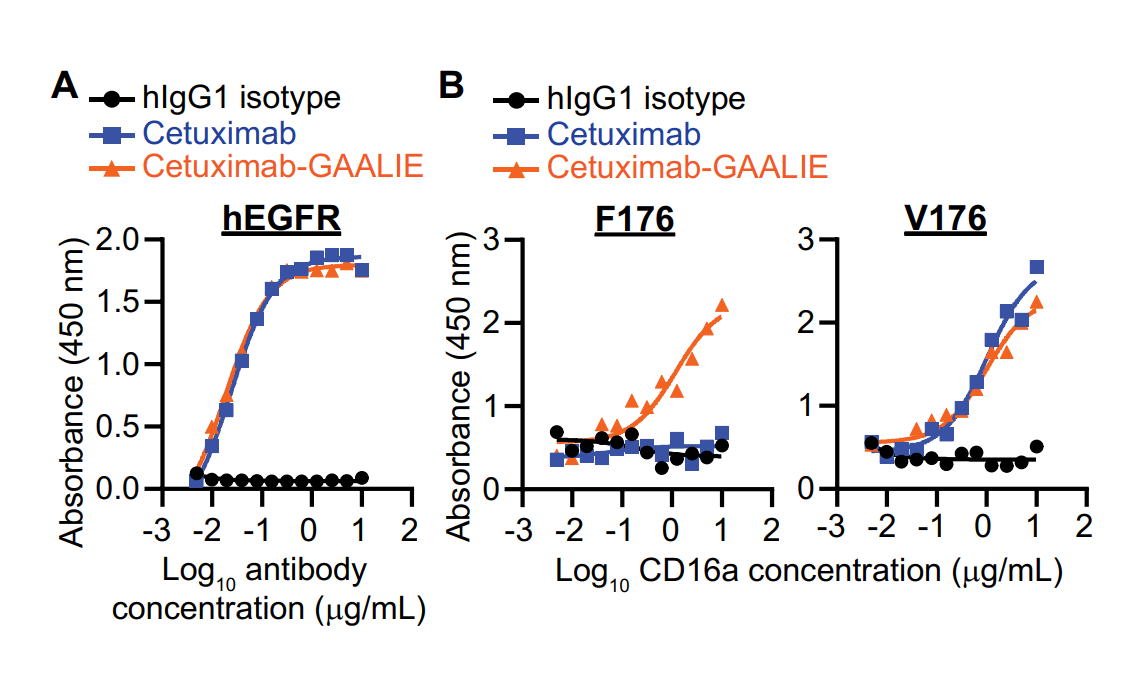
ichorbio has recently introduced a GAALIE version of cetuximab.
Data from the Ferrari de Andrade lab at Icahn School of Medicine at Mount Sinai demonstrates that cetuximab-GAALIE binds to human EGFR similarly compared to wild type cetuximab, and most importantly that cetuximab-GAALIE binds with higher affinity to CD16a F176.