Basiliximab: A Comprehensive Overview
1. Background Study
Kidney transplantation is a surgery-based treatment for patients who have prolonged kidney failure or are suffering from chronic or end-stage kidney failure (CKD) [1]. Kidney transplant (KT) procedures in older patients rise daily [2]. The National Kidney Foundation showed that 786,000 patients are living with kidney problems, but only around 25,000 received transplants during 2021 [3]. In the United Kingdom, 2868 adult kidney transplants are performed, and approximately 5,000 patients should have gone through this process [4]. However, 10 to 15 patients’ kidneys are rejected in every 100 patients during the first year after a kidney transplant [5]. Therefore, an immunosuppressive agent like Basiliximab significantly reduces transplanted kidney rejection [6].
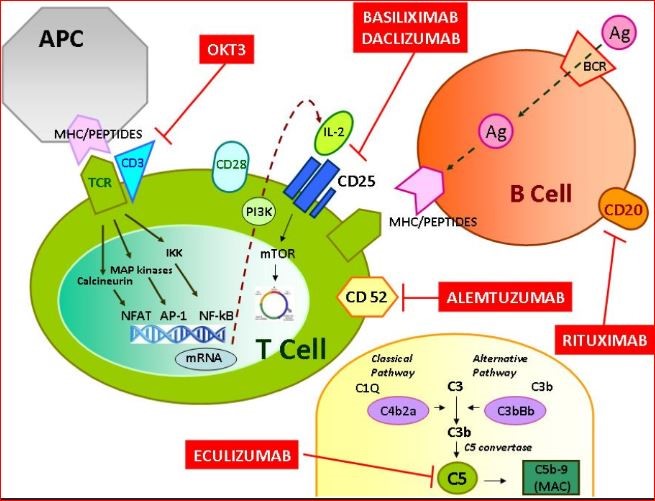
2. Target and its mechanism
Basiliximab, a chimeric monoclonal antibody and glycoprotein developed by recombinant technique, prevents white blood cells (WBC) from the risky rejection of kidneys after transplantation in the human body [6, 7]. It explicitly targets the alpha chain of interleukin-2 (IL-2) receptor or Cluster of differentiation 25 antigen (CD25) with high specificity on the surface of triggered T-lymphocytes [8]. Then, Basiliximab blocks IL-2-mediated stimulation and proliferation of T-cells binding with CD25, significantly contributing to the down-regulation of allograft rejection in transplanted kidneys [9].
3. Medicinal usages
In order to inhibit effectively transplanted kidneys in human bodies, the FDA (Food and Drug Administration) approved Basiliximab as an antibody medication for kidney transplantation in 1998 [10, 11]. Patients are often prescribed two doses: the first dose is administrated two hours prior to the initiation of the procedure, and the second dose is given four days following a successful transplantation [12].
4. Clinical study results
Current clinical trials have demonstrated the efficacy and safety of Basiliximab in kidney transplantation. It meaningfully reduces the acute rejection occurrence and moderates the survival rate of graft-kidney patients compared to placebo therapy, according to a randomized controlled study [13]. Another study illustrated that patients’ and graft survival rates are 98% and 94%, respectively, including only a 9.6% rejection rate with Basiliximab management throughout one year. Furthermore, 29% of kidney patients with Basiliximab need dialysis after the transplantation process, in comparison with antithymocyte globulin with 30% (a polyclonal antibody) [14].
5. Side effects
Basiliximab intake usually does not cause serious problems in patients [13]. However, some minor side effects, including gastrointestinal disorders, fever, fatigue, anorexia, skin rash, headache, and haematological abnormalities, are found. Severe detrimental difficulties, such as acute hypersensitivity, anaphylaxis, and capillary leak syndrome, are rarely observed due to unusual consumption [15].
6. Molecular engineering and development
Basiliximab is genetically engineered using a recombinant DNA technique from a mouse myeloma cell line. It is a chimeric antibody that includes the entire murine variable region and approximately 80% human [7]. Using chimeric antibodies reduces the risk of immunogenicity and improves the pharmacokinetic properties compared to fully murine antibodies [16].
7. Potential drug interactions
Moreover, it is more likely that infections and other side effects will occur due to the interaction of Basiliximab with other immunosuppressive drugs, such as corticosteroids and calcineurin inhibitors [17]. Adult patients who took Basiliximab with calcineurin inhibitors during all four days of treatment had higher levels of tacrolimus trough than those control patients who did not take the drug [18]. So, while taking Basiliximab in addition to another immunosuppressant, careful monitoring of the levels of the immunosuppressive medication and dose modifications may be required [19].
8. New prospective uses
Current studies also report using Basiliximab for solid organ transplants, such as liver and heart transplants. Furthermore, considering that Basiliximab helps manage T-cell activation, a treatment option for autoimmune diseases, e.g., rheumatoid arthritis and inflammatory bowel disease, has been studied [22, 23]
9. Other antibodies in clinical development
Basiliximab is not the only option for such antibodies; several monoclonal antibodies acting on the IL-2 pathway and used during transplantation and for other autoimmune diseases are under clinical trials [24, 25]. Furthermore, according to the results of classic research, some antibodies that are likely to block the action of co-stimulatory pathways, including CD28/B7, are also promising alternatives to Basiliximab [26].
Ichorbio is a high-quality manufacturer of biosimilars and has developed a Basiliximab biosimilar. Find the product: https://ichor.bio/basiliximab-biosimilar-research-grade-ich5189
References:
1. Abramyan S, Hanlon M. Kidney Transplantation. [Updated 2023 Jan 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567755/ [Extracted Information on 12 April 2024]
2. Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: kidney. Am J Transplant. 2018;18 Suppl 1:18–113.
3. https://www.kidney.org/newsletter/transplants-all-saving-lives-one-kidney-time [Extracted Information on 12 April 2024]
4. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/27956/kidney-annual-report-2021-22.pdf [Extracted Information on 12 April 2024]
5. https://www.nhsbt.nhs.uk/organ-transplantation/kidney/benefits-and-risks-of-a-kidney-transplant/risks-of-a-kidney-transplant/rejection-of-a-transplanted-kidney/#:~:text=Rejection%20happens%20in%20between%2010,proteins%20('antibodies'). [Extracted Information on 12 April 2024]
6. López-Abente, J., Martínez-Bonet, M., Bernaldo-de-Quirós, E. et al. Basiliximab impairs regulatory T cell (TREG) function and could affect the short-term graft acceptance in children with heart transplantation. Sci Rep 11, 827 (2021).
7. Salis P, Caccamo C, Verzaro R, Gruttadauria S, Artero M. The role of basiliximab in the evolving renal transplantation immunosuppression protocol. Biologics. 2008 Jun;2(2):175-88.
8. Kapic E, Becic F, Kusturica J. Basiliximab, mechanism of action and pharmacological properties. Med Arh. 2004;58(6):373-6.
9. Short S, Lewik G, Issa F. An Immune Atlas of T Cells in Transplant Rejection: Pathways and Therapeutic Opportunities. Transplantation. 2023 Nov 1;107(11):2341-2352. doi: 10.1097/TP.0000000000004572. Epub 2023 Oct 21.
10. Martin, F., Xiao, Y., Welten, V., Nakamori, K., Gizlenci, M., Zhou, H., & Tullius, S. G. (2024). The combinatorial effect of age and biological sex on alloimmunity and transplantation outcome. Frontiers in Transplantation, 2, 1325232.
11. Willoughby LM, Schnitzler MA, Brennan DC, et al. Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: application of statisticalapproaches to reduce bias in observational comparisons. Transplantation. 2009; 87:1520–9.
12. Li J, Li X, Tan M, Lin B, Hou S, Qian W, Li B, Zhang D, Zhou B, Wang H, Zhu T, Guo Y. Two doses of humanized anti-CD25 antibody in renal transplantation: a preliminary comparative study. MAbs. 2009 Jan-Feb;1(1):49-55.
13. Ponticelli, C. (2014). Basiliximab: efficacy and safety evaluation in kidney transplantation. Expert Opinion on Drug Safety, 13(3), 373–381.
14. Antithymocyte globulin, Editor(s): J.K. Aronson, Meyler's Side Effects of Drugs (Sixteenth Edition), Elsevier, 2016, Pages 626-630, ISBN 9780444537164, https://doi.org/10.1016/B978-0-444-53717-1.01671-1.
15. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Basiliximab. [Updated 2017 Sep 21]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548587/ [Extracted Information on 12 April 2024]
16. Vaisman-Mentesh A, Gutierrez-Gonzalez M, DeKosky BJ, Wine Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-Drug Antibody Formation Following Treatment with Monoclonal Antibodies. Front Immunol. 2020 Aug 18; 11:1951. doi: 10.3389/fimmu.2020.01951.
17. Bestard O, Thaunat O, Bellini MI, Böhmig GA, Budde K, Claas F, Couzi L, Furian L, Heemann U, Mamode N, Oberbauer R, Pengel L, Schneeberger S, Naesens M. Alloimmune Risk Stratification for Kidney Transplant Rejection. Transpl Int. 2022 May 20; 35:10138.
18. Sifontis NM, Benedetti E, Vasquez EM. Clinically significant drug interaction between basiliximab and tacrolimus in renal transplant recipients. Transplant Proc. 2002; 34:1730–2.
19. Shemshadi M, Hoseini R, Zareh R, Otukesh H. Use of Basiliximab with the Standard Immunosuppressive Protocol in Pediatric Renal Transplantation: A Double-Blind Randomized Clinical Trial. Int J Organ Transplant Med. 2020;11(1):8-14.
20. Hashim M, Alsebaey A, Ragab A, Soliman HE, Waked I. Efficacy and safety of basiliximab as initial immunosuppression in liver transplantation: A single center study. Ann Hepatol. 2020 Sep-Oct;19(5):541-545. doi: 10.5604/01.3001.0012.2246. Epub 2020 Aug 5.
21. Rudzik KN, Rivosecchi RM, Palmer BA, Hickey GW, Huston JH, Keebler ME, Kaczorowski DJ, Horn ET. Basiliximab induction versus no induction in adult heart transplantation. Clin Transplant. 2023 May;37(5): e14937. doi: 10.1111/ctr.14937. Epub 2023 Feb 26.
22. Brumby C, Huang L, Lee D, McMahon L. Acute polyarthritis immediately after kidney transplantation: a medication-induced rheumatoid arthritis flare? Nephrology (Carlton). 2014 Apr;19 Suppl 1:2-5. doi: 10.1111/nep.12190.
23. Kiskaddon AL, Wilsey M, Gonzalez-Gomez I, Laks J, Miles A, Carapellucci J, Asante-Korang A. Basiliximab therapy for immune-mediated bowel disease in a pediatric heart transplant patient. Pediatr Transplant. 2023 Mar;27(2): e14443. doi: 10.1111/petr.14443. Epub 2022 Nov 23.
24. Cohan SL, Lucassen EB, Romba MC, Linch SN. Daclizumab: Mechanisms of Action, Therapeutic Efficacy, Adverse Events and Its Uncovering the Potential Role of Innate Immune System Recruitment as a Treatment Strategy for Relapsing Multiple Sclerosis. Biomedicines. 2019 Mar 11;7(1):18.
25. Li M, Sun K, Welniak LA, Murphy WJ. Immunomodulation and pharmacological strategies in the treatment of graft-versus-host disease. Expert Opin Pharmacother. 2008 Sep;9(13):2305-16. doi: 10.1517/14656566.9.13.2305.
26. van der Zwan M, Hesselink DA, van den Hoogen MWF, Baan CC. Costimulation Blockade in Kidney Transplant Recipients. Drugs. 2020 Jan;80(1):33-46.


